Which one of the following compounds on treatment with LiAlH4 will give a product that will give a positive iodoform test?
1. CH3CH2CHO
2. CH3CH2COOCH3
3. CH3CH2OCH2CH3
4. CH3COCH3
Aldehydes and ketones will not form crystalline derivatives with
1. sodium bisulphite
2. phenyl hydrazine
3. semicarbazide hydrochloride
4. dihydrogen sodium phosphate
Oxalic acid on treatment with conc. H2SO4 gives:
1. CO + H2O2
2. H2O +CO+CO2
3. HCOOH + CO2
4. HCOOH + CO2 +O2
Benzene sulphonic acid on treating with P2O5 gives:
1. salicylic acid
2. benzoic acid
3. acid anhydride
4. sodium benzoate
The IUPAC name of crotonaldehyde is:
1. But-2-en-1-al
2. Prop-2-enal
3. 4-Methylpent-3-en-2-one
4. None of the above
The IUPAC name of tartaric acid is:
1. 2,3-dihydroxy butane-1, 4-dioic acid
2. 1,4-dihydroxy butane-2-3-dioic acid
3. butane-1 4-dicarboxylic acid
4. none of the above
Cyanohydrin of which compound gives lactic acid on hydrolysis?
1. Acetone
2. Acetaldehyde
3. Propanal
4. HCHO
Which of the following is the strongest acid?
1. HCOOH (pKa 3.77)
2. C6H5COOH (pKa 4.22)
3. CH3COOH (pKa 4.71)
4. CH3CH2COOH (pKa 4.88)
Which of the following would produce secondary alcohol?
1. C6H5COCH3
2. C6H5COCH3
3. C6H5CHO
4. C6H5COOMe


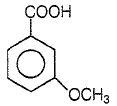
Product P in the above reaction is -
1. 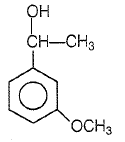

2. 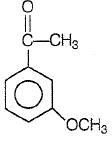

3. 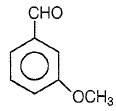

4.