In a reversible reaction, a catalyst:
1. increases the rate of the forward reaction only
2. increases the rate of the forward reaction to a greater extent than that of the backward reaction
3. increases the rate of the forward reaction and decreases that of the backward reaction to the different extent
4. increases the rate of the forward and backward reactions equally
Gas masks containing activated charcoal remove poisonous gases from the atmosphere, and act on the principle of:
1. Adsorption
2. Absorption
3. Sorption
4. All of these
Tanning of leather is:
1. coloring of leather by chemicals
2. drying process to make the leather hard
3. polishing of leather to make it look attractive
4. coagulative hardening of the leather by chemicals
When sulphur sol is evaporated solid sulphur is left. On mixing with water no colloidal sol is formed. Sulphur sol is:
1. hydrophilic
2. hydrophobic
3. reversible
4. lyophilic
The Critical Micelle Concentration (CMC) is:
1. The concentration at which micellization starts
2. The concentration at which true solution is formed
3. The concentration at which one molar electrolyte is present per 1000 g of solution
4. The concentration at which solute and solution from equilibrium
In adding 1 mL of solution of 10 % NaCl to 10 mL of gold solution in the presence of 0.25 g of starch, the coagulation is just prevented. Starch has a gold number equal to:
1. 0.25
2. 2.5
3. 250
4. 0.025
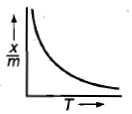
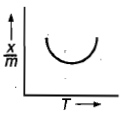
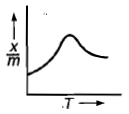
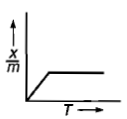
Which plot is the adsorption isobar for chemisorption where X is the amount of gas adsorbed on mass m (at constant pressure) at temperature T
1. 
2. 
3. 
4. 
What is a solution that isn't a lyophilic colloid?
1. Milk
2. Gum
3. Fog
4. Blood
The surface tension of lyophilic sols is:
1. Lower than H2O
2. More than H2O
3. Equal to H2O
4. None of the above.
Hardy-Schulze rule states that:
1. non-electrolytes have better coagulating action on colloids than electrolytes
2. sols are coagulated by effective ions whose charge is opposite to that of sol and the ions of higher charge are much more effective than the ions of lower charge
3. charge of the ions has no effect on the coagulation of a sol
4. sols are coagulated only by those ions whose charge is similar to that of the sol
