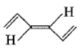
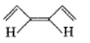
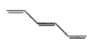
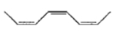
A paramagnetic species among the following is-
1.
A carbocation
2.
A free radical
3.
A carbanion ion
4.
All of the above
A student named the compound as 1,4-butadiene:
1. The name is correct
2. He committed an error in the selection of carbon chain
3. He committed an error in the position of the double bond
4. Unpredictable
The IUPAC name of ,

1. 2-Chloromethyl-4-methyl-hexanal
2. 1-Chloro-4-ethyl-2-pentanal
3. 1-Chloro-4-methyl-2-hexanal
4. 1-Chloro-2-aldo-4-methylhexane
The IUPAC name of the compound is-
\(CH_2=C-CH_2-C \equiv CH\\ ~~~~~~~~~~~~~|\\ ~~~~~~~~~~~~CH_3\)
1. 2-Methylpent-1-en-4-yne
2. 4-Methylpent-4-en-1-yne
3. 2-Methylpent-2-en-4-yne
4. 4-Methylpent-1-en-4-yne
IUPAC name of, CH3CH(OH)CH2CH2COOH is-
1. 4-Hydroxypentanoic acid
2. 1-Carboxy-3-butanoic acid
3. 1-Carboxy-4-butanol
4. 4-Carboxy-2-butanol
IUPAC name of the compound, ClCH2CH2COOH is-
1. 3-Chloropropanoic acid
2. 2-Chloropropanoic acid
3. 2-Chloroethanoic acid
4. Chlorosulfonic acid
IUPAC suffix name of -COX is-
1. oyl halide
2. Halocarbonyl
3. Carbamoyl
4. None of the above
IUPAC name of C6H5CN is-
1. Phenyl nitrile
2. Benzonitrile
3. Benzyl nitrile
4. Phenyl cyanide
IUPAC name of the compound, is-
1. But-2-en-1-ol
2. 1-Hydroxy but-1-ene
3. 4-Hydroxy butene-3
4. But-1-en-1-ol