In following reaction, find out X and Y.
1. X=2-butyne ; Y=3-hexyne
2. X=2-butyne ; Y=2-hexyne
3. X=1-butyne ; Y=2-hexyne
4. X=1-butyne ; Y=3-hexyne
An organic compound X having molecular formula C5H10O yields phenyl hydrazone and
gives negative response to the iodoform test and Tollen's test. It produces n-pentane on
reduction. X could be
1. pentenal
2. 2-pentanone
3. 3-pentanone
4. n-amyl alcohol
Which of the following will not be soluble in sodium hydrogen carbonate?
1. 2, 4,6 - trinitrophenol
2. benzoic acid
3. o-nitrophenol
4. Benzenesulphonic acid
In a reaction,
A is
1. HgSO4/H2SO4
2. Cu2Cl2
3. H3PO2 and H2O
4. H+/H2O
In the following sequence of reactions,
CH3-Br ABC
the end product C is
1. acetone
2. methane
3. acetaldehyde
4. ethyl alcohol
CH3CHO and C6H5CH2CHO can be distinguished chemically by
1. Benedict test
2. iodoform test
3. tollen's reagent test
4. Fehling solution test
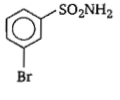
In a set of reactions, m-bromobenzoic acid gave a product D. Identify the product D.

1.
2.
3.
4. 
What is the product obtained in the following reaction?
Which one is a nucleophilic substitution reaction among the following?
1.
2.
3.
4.
The reaction of toluene with Cl2 in presence of FeCl3 gives 'X' and reaction in presence of
light gives 'Y'. Thus, 'X' and 'Y' are
1. X = Benzal chloride, Y = o-chlorotoluene
2. X = m-chlorotoluene, Y= p-chlorotoluene
3. X = o-and p-chlorotoluene, Y=trichloromethyl benzene
4. X = Benzyl chloride, Y = m-chlorotoluene






