Volume, pressure, and temperature of an ideal gas are \(V\), \(P\), and \(T\) respectively. If the mass of its molecule is \(m\), then its density is: [\(k\)=Boltzmann's constant]
1.
\(mkT\)
2.
\(P \over kT\)
3.
\(P \over kTV\)
4.
\(Pm \over kT\)
One liter of gas A and two liters of gas B, both having the same temperature 100C and the same pressure 2.5 bar will have the ratio of average kinetic energies of their molecules as:
1. 1:1
2. 1:2
3. 1:4
4. 4:1
On 0C, the pressure measured by the barometer is 760 mm. What will be pressure at 100C? [AFMC 2002]
1. 760 mm
2. 730 mm
3. 780 mm
4. None of these
By what percentage, should the pressure of a given mass of gas be increased, so as to decrease its volume by 10% at a constant temperature?
1. 5%
2. 7.2 %
3. 12.5%
4. 11.1%
In the adjacent V-T diagram what is the relation between ?
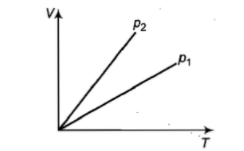
1.
2.
3.
4. cannot be predicated
Which one of the following graph is correct at constant pressure?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The root-mean-square velocity of the molecules in a sample of helium is of that of the molecules in a sample of hydrogen. If the temperature of the hydrogen gas is 0C, that of the helium sample is about:
1. 0C
2. 5.6C
3. 273C
4. 100C
The kinetic energy of one gram molecule of a gas at standard temperature and pressure is: (R = 8.31 J/mol-K)
1. 0.56
2.
3.
4.
Gases exert pressure on the walls of containing vessel because the gas molecules:
1. Possess momentum
2. collide with each other
3. have finite volume
4. obey gas laws
The equation of state for 5 g of oxygen at a pressure P and temperature T, when occupying a volume V, will be: (where R is the constant)
1. PV = 5RT
2. PV =
3. PV =
4. PV =
