The correct statement about the SN1 reaction is:
| 1. | 100 % racemisation. |
| 2. | Inversion more than retention leads to partial racemisation. |
| 3. | 100 % retention. |
| 4. | 100 % Inversion. |
Which one of the following statements is not correct for a nucleophile?
1. Nucleophile is a lewis acid.
2. Ammonia is a nucleophile.
3. Nucleophiles attack low electron density sites.
4. Nucleophiles are not electron-seeking.
Two possible stereo-structures of CH3CHOH.COOH, that are optically active, called:
| 1. | Diastereomers | 2. | Atropisomers |
| 3. | Enantiomers | 4. | Mesomers |
In Duma's method for estimation of nitrogen, 0.25 g of an organic compound gave 40 mL of nitrogen collected at 300 K temperature and 725 mm pressure. If the aqueous tension at 300 K is 25 mm, the percentage of nitrogen in the compound is:
| 1. | 17.36 | 2. | 18.20 |
| 3. | 16.76 | 4. | 15.76 |
In which of the following compounds, the C—Cl bond ionization shall give the most stable carbonium ion?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Hyperconjugation occurs among the following compounds:
| 1. | I only | 2. | II only |
| 3. | III only | 4. | I and III |
The enolic form of ethyl acetoacetate is given below:
The number of σ and π bonds in the enolic form are respectively:
1. 18 sigma bonds and 2 pi-bonds
2. 16 sigma bonds and 1 pi-bond
3. 9 sigma bonds and 2 pi-bonds
4. 9 sigma bonds and 1 pi-bond
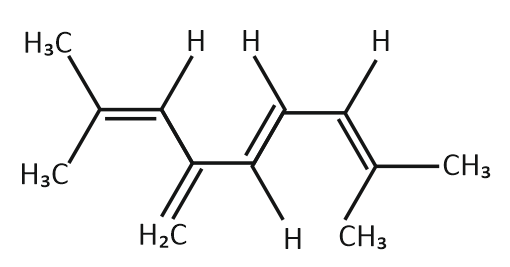
| 1. | Four (4) | 2. | Eight (8) |
| 3. | Twelve (12) | 4. | Sixteen (16) |
In Kjeldahl’s method for estimation of nitrogen present in the soil sample, ammonia evolved from 0.75g of sample neutralized 10ml of 1M H2SO4. The percentage of nitrogen in the soil is:
| 1. | 37.33 | 2. | 45.85 |
| 3. | 25.75 | 4. | 43.13 |
Which structure corresponds to the IUPAC name 3-Ethyl-2-hydroxy-4-methylhex-3-en-5-ynoic acid?
| 1. |  |
2. |  |
| 3. |  |
4. |  |


