|
A |
B |
|
A. |
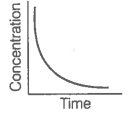
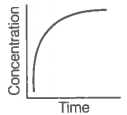
i. Variation in product concentration with time. |
|
B. |
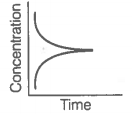
ii. Reaction at equilibrium |
|
C. |
iii. Variation in reactant concentration with time. |
Match the graphical variations with their descriptions given above and identify the correct codes below:
| A | B | C | |
| 1. | i | iii | ii |
| 2. | i | ii | iii |
| 3. | iii | ii | i |
| 4. | iii | i | ii |
The role of a catalyst is to change:
| 1. | Gibbs energy of the reaction |
| 2. | Enthalpy of reaction |
| 3. | The activation energy of the reaction |
| 4. | Equilibrium constant |
In the presence of a catalyst, the heat evolved or absorbed during the reaction:
| 1. | Increases. | 2. | Decreases. |
| 3. | Remains unchanged. | 4. | May increase or decrease. |
| 1. | Determining the rate constant at standard temperature. |
| 2. | Determining the rate constant at two temperatures. |
| 3. | Determining probability of collision. |
| 4. | Using the catalyst. |
The correct statement based on the graph below is:

| 1. | The activation energy of the forward reaction is E1 + E2 and the product is less stable than reactant. |
| 2. | The activation energy of the forward reaction is E1 + E2 and the product is more stable than the reactant. |
| 3. | The activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than the product. |
| 4. | The activation energy of the backward reaction is E1 and the product is more stable than reactant. |
Consider the first-order gas-phase decomposition reaction given below.
A(g) → B(g) + C(g)
The initial pressure of the system before the decomposition of A was . After the lapse of time t, the total pressure of the system increased by X units and became . The rate constant k for the reaction is:
| 1. | 2. | ||
| 3. | 4. |
The correct graphical representation of relation between ln k and 1/T is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Consider the Arrhenius equation given below and choose the correct option:
| 1. | Rate constant increases exponentially with increasing activation energy and decreasing temperature. |
| 2. | Rate constant decreases exponentially with increasing activation energy and increasing temperature. |
| 3. | Rate constant increases exponentially with decreasing activation energy and decreasing temperature. |
| 4. | Rate constant increases exponentially with decreasing activation energy and increasing temperature. |
A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in the graph below.

The correct statement among the following based on the graph given above is:
Which of the following statements is not correct about the order of a reaction?
| 1. | The order of a reaction can be a fractional number |
| 2. | Order of a reaction is experimentally determined quantity |
| 3. | The order of a reaction is always equal to the sum of the stoichiometric coefficients of reactants in the balanced chemical equation for a reaction |
| 4. | The order of a reaction is the sum of the powers of the molar concentration of the reactants in the rate law expression |