Q. 46 Using crystal field theory, draw energy level diagram, wrile electronic
configuration of the central metal atom/ion and determine the magnetic
moment value in the following.
(a)
(b)
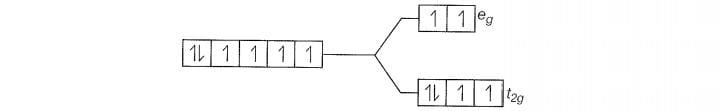
F is a weak field ligand.
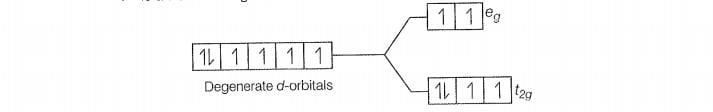
Configuration of Co
Number of unpaired electrons (n) =4
Magnetic moment ()=
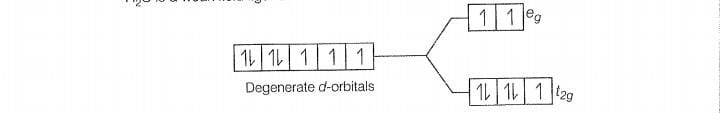
Configuration of Co
There is no unpaired electon, so it is diamagnetic
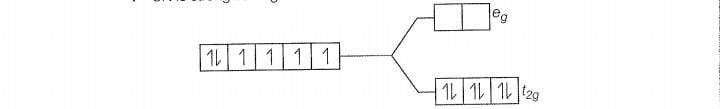
(b)
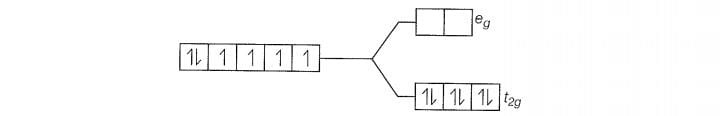
Since , CN is a strong filed ligand, all the electrons get paired.
Because there is no unpaired electron, so it is diamagnetic in nature.

© 2026 GoodEd Technologies Pvt. Ltd.