4.11 The following results have been obtained during the kinetic studies of the reaction:
2A + B → C + D
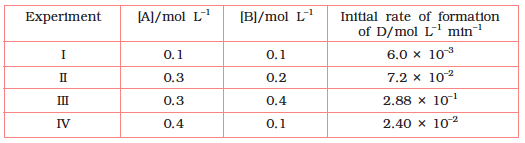
Determine the rate law and the rate constant for the reaction.
Let the order of the reaction with respect to A be x and with respect to B be y.
Therefore, rate of the reaction is given by,
Dividing equation (iv) by (i), we obtain
Dividing equation (iii) by (ii), we obtain
Therefore, the rate law is
Rate = k [A] [B]
From experimental I, we obtain
From experimental II, we obtain
From experimental III, we obtain
From experimental IV, we obtain
Therefore, rate constant, k =

© 2026 GoodEd Technologies Pvt. Ltd.