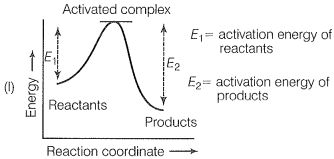
The correct representation of an exothermic reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. | Both 1 and 2 |
HINT: Reactant energy is more than product energy formexothermic reaction.
Explanation: The chemical reaction in which energy is evolved during the reaction is exothermic known as exothermic reaction i.e. activation energy of the product is greater than the activation energy of reactants.
Here, only (1) denotes the correct picture of the exothermic reaction.

© 2026 GoodEd Technologies Pvt. Ltd.