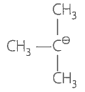
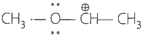Match the ions given in Column I with their nature given in Column II.
| Column I (Ions) |
Column II (Corresponding nature) |
||
| A. |  |
I. | Stable due to resonance |
| B. |  |
II. | Destabilised due to inductive effect |
| C. |  |
III. | Stabilised by hyperconjugation |
| D. |  |
IV. | A secondary carbocation |
Codes:
| Options: | A | B | C | D |
| 1. | I,II | II | II | III,IV |
| 2. | I | II,IV | III | IV |
| 3. | I | IV | III | II |
| 4. | IV | I | III | II |
|
Column I |
Column II |
Explanation |
|
A. |
Stable due to resonance Destabilised due to inductive effect |
|
|
B. |
Destabilised due to inductive effect |
-/effect of F creates electron deficiency at carbon C+ |
|
C. |
Destabilised due to inductive effect |
+/effect of CH3 increases electron density at carbon C- |
|
D. |
A secondary carbocation stabilized due to hyperconjugation |
is attached to two carbon. It can also be stabilized by hyperconjugation. |

© 2026 GoodEd Technologies Pvt. Ltd.