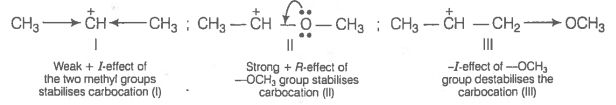
The correct order of decreasing stability of the following carbocations is:
| I | \( \begin{aligned} &~~~~~~~~~~~~\oplus\\ & CH_3 - CH-CH_3 \end{aligned}\) |
| II | \( \begin{aligned} &~~~~~~~~~~~~\oplus\\ & CH_3 - CH-OCH_3\end{aligned}\) |
| III | \( \begin{aligned} & ~~~~~~~~~~~~\oplus\\ &CH_3 - CH-CH_2- OCH_3\end{aligned}\) |
| 1. | II > I > III | 2. | I > II > III |
| 3. | II < I < III | 4. | I < II < III |

© 2026 GoodEd Technologies Pvt. Ltd.