Given below are two statements:
| Assertion (A): | Though the central atom of both NH3 and H2O molecules are sp3 hybridised, yet H-N-H bond angle is greater than that of H-O-H. |
| Reason (R): | This is because nitrogen atom has one lone pair and oxygen atom has two lone pairs. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
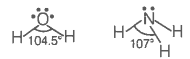

© 2026 GoodEd Technologies Pvt. Ltd.