
Product (C) is
1. Nitrobenzene
2. 1,3-Diethoxybenzenez
3. Ethoxybenzene
4. Benzene
The major product of the following reaction would be
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Which of the following is regenerated at the end of the reaction
(1) X
(2) Y
(3) Z
(4) W
'x' and 'y' are respectively :
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
What will be the product of the following reaction
| (1) |  |
| (2) |  |
| (3) |  |
| (4) |  |
Product (A) is:
1.
2.
3.
4.

1.
2.
3.
4.
(A) +

A will be:
(a)
(b)
(c)
(d)
The product formed in the below mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Above compounds (P) & (Q) can be differentiated by:
(1) amm. AgNO3
(2) NaOH
(3) FeCl3
(4) Both (a) & (b)
Gas evolved during the reacton of sodium metal on ethyl-amine is:
(1) N2
(2) C2H2
(3) H2
(4) CO2
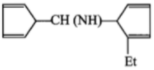
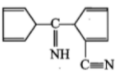
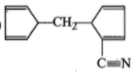
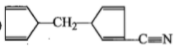
The product [A] formed in the reaction;
1. 
2. 
3. 
4. 
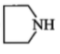
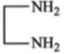
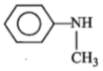
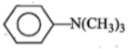
ln the reaction,
The product is:
1. 
2. 
3. 
4. 
A primary amine heated with CS2 in presence of excess of HgCl2 gives isothiocyanate. The reaction is called:
(1) Hofmann's bromamide reaction
(2) Hofmann's mustard oil reaction
(3) Perkin's condensation
(4) Hofmann's elimination
Reaction of cyclohexanone with dimethylamine in the presence of catalytic amount of an acid forms a compound if water during the reaction is continuously removed. The compound formed is generally known as:
(1) Schiff's base
(2) an enamine
(3) an imine
(4) an amine
Biuret test is not given by:
(1) proteins
(2) carbohydrates
(3) polypeptides
(4) urea
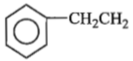
The product formed by the reaction of C6H5CN and CH2N2 is:
1. 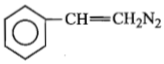
2.
3. 
4. None of these
The presence of radical in solution can be detected by:
1. Fehling’s solution
2. Benedict’s solution
3. Schiff's reagent
4. Nessler’s reagent
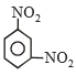
A given nitrogen-containing aromatic compound a reacts with Sn/HCl, followed by HNO2 to give an unsatable compound B.B, on treament with phenol, forms a beautiful coloured compound C with the molecular formula C12H10N2O. The structure of compound A is
The electrolytic reduction of nitrobenzene in a strongly acidic medium produces
1. p-Aminophenol
2. Azoxybenzene
3. Azobenzene
4. Aniline
Method by which aniline cannot be prepared is
1. hydrolysis phenyl isocyanide with an acidic solution
2. degradation of benzamide with bromine in alkaline solution
3. reduction of nitrobenzene with H2/Pd in ethanol
4. potassium salt of phthalimide treated with chlorobenzene followed by the hydrolysis
with aqueous NaOH solution