The number of (i) sp2 hybridized carbon atoms and (ii) bonds are present in the following compound are: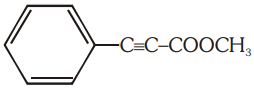
1. 7, 5
2. 8, 6
3. 7, 6
4. 8, 5
From the following pairs, the two species that are not isostructural are :
| 1. | \(\mathrm{PF}_5~\mathrm{and}~\mathrm{BrF}_5\) | 2. | \(\mathrm{PCl}^+_4~\mathrm{and}~\mathrm{SiCl}_4\) |
| 3. | \(\mathrm{CO}_{3}^{2-} \ \mathrm{and} \ \mathrm{NO}_{3}^-\) | 4. | \(\mathrm{AIF}^{3-}_6~\mathrm{and}~\mathrm{SF}_6\) |
The specie with the shortest bond length is:
| 1. | \(O^+_2\) | 2. | \(O^-_2\) |
| 3. | \(O^{2-}_2\) | 4. | \(O^{2+}_2\) |
The correct increasing order of the polarizing power of the cationic species is:
1.
2.
3.
4.
The number and type of bonds between two carbon atoms in calcium carbide are
1. One sigma, two pi
2. Two sigma, two pi
3. One sigma, one pi
4. Two sigma, one pi
The extent of lattice energy in an ionic compound depends on:
1. Packing of ions only
2. Size of the ions only
3. Charge and Size of the ions
4. Charge on the ions only
A molecule having the smallest bond angle among the following is :
| 1. | \(\mathrm{SO}_2\) | 2. | \(\mathrm{H_2O}\) |
| 3. | \(\mathrm{H_2S}\) | 4. | \(\mathrm{NH}_3\) |
The hybridization of orbitals of N atom in are respectively:
1.
2.
3.
4.
The number of hydrogen bonded water molecules(s) associated with \(\mathrm{C u S O_{4} . 5 H_{2} O}\) is:
| 1. | 3 | 2. | 1 |
| 3. | 2 | 4. | 5 |
The ionic species that has the greatest proton affinity to form stable compound is:
1.
2.
3.
4.
The correct order of C-O bond length among CO, , CO2 is:
1.
2.
3.
4.
Isostructural pair among the following is:
1.
2.
3.
4.
| 1. | BeH2 < CaH2 < BaH2 | 2. | CaH2 < BeH2 < BaH2 |
| 3. | BeH2 < BaH2 < CaH2 | 4. | BaH2 < BeH2 < CaH2 |
Which bond angle would result in the maximum dipole moment for the triatomic molecule
1.
2.
3.
4.