A hydrogenation reaction is carried out at 500 K. If the same reaction is carried out in the presence of a catalyst at the same rate with same frequency factor, the temperature required is 400 K. If the catalyst lowers the activation energy barrier by 16 kJ/mol then the activation energy of reaction is:
(1) 100 kJ/mol
(2) 80 kJ/mol
(3) 60 kJ/mol
(4) None of these
Consider the reaction.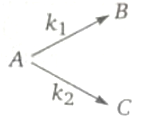
The rate constant for two parallel reactions were found to be and . If the corresponding energy of activation of the parallel reaction are 100 and 120 kJ/mol respectively, The net energy of activation of A is:
(1) 150 kJ/mol
(2) 320 kJ/mol
(3) 116 kJ/mol
(4) 220 kJ/mol
A reaction takes place in various steps. The rate constant for first, second, third and fifth steps are respectively. The overall rate constant is given by ,
If activation energy are 40, 60, 50 and 10 kJ/mol respectively, the overall energy of activation (kJ/mol) is:
(1) 10
(2) 20
(3) 25
(4) None of the above.
For first order parallel reactions are 4 min-1 and 2 respectively at 300 K. If the activation energies for the formation of B and C are respectively 30,000 and 38,314 J/mol respectively, the temperature at which B and C will be obtained in equimolar ratio is: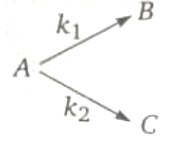
(1) 757.48 K
(2) 378.74 K
(3) 600 K
(4) None of the above
For reaction AB, the rate constant and for the reaction XY, the rate constant . If , then the temperature at which is (Given: R=2 cal/K-mol)
(1) 1200 K
(2) 12004.606 K
(3)
(4)
Consider the plots, given below, for the types of reaction
nAB+C

These plots respectively correspond to the reaction orders:
1. 0, 1, 2
2. 1, 2, 0
3. 1, 0, 2
4. None of the above
A graph between log and log a (abscissa), a being the initial concentration of A in the reaction for reaction A Product, the rate law is: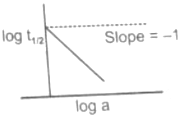
(1)
(2)
(3)
(4)
For a zero order reaction, the plot of conc. (a-x) vs time is linear with
(1) +ve slope and zero intercept
(2) -ve slope and zero intercept
(3) +ve slope and non-zero intercept
(4) -ve slope and non-zero intercept
The kinetic data for the given reaction A(g) + 2B(g) C(g) is provided in the following table for three experiments at 300 K
| Ex. No. | [A/M] | [B/M] | Initial rate (M sec-1) |
| 1. | 0.01 | 0.01 | 6.930 |
| 2. | 0.02 | 0.01 | 1.386 |
| 3. | 0.02 | 0.02 |
1.386 |
In another experiment starting with initial concentration of 0.5 and 1 M respectively for A and B at 300 K, find the rate of reaction after 50 minutes from the starting of experiment (in M/sec).
(1)
(2)
(3)
(4)
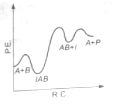
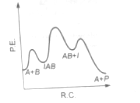
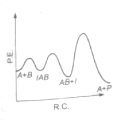
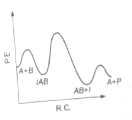
The following mechanism has been proposed for the exothermic catalyzed complex reaction
If is much smaller than , the most suitable qualitative plot of potential energy (P.E.) versus reaction co-ordinate (R.C.) for the above reaction
(1) 
(2) 
(3) 
(4) 
The reaction A(g) + 2B(g)C(g) is an elementary reaction. In an experiment involving this reaction, the initial partial pressures of A and B are respectively. When atm, the rate of the reaction relative to the initial rate is:
(1)
(2)
(3)
(4) None of these
The forward rate constant for the elementary reversible gaseous reaction
What is the rate constant for the backward reaction at this temperature if moles of and 10 moles of are present in a 10 litre vessel at equilibrium.
(1)
(2)
(3)
(4)
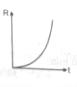
If decomposition reaction A(g)B(g) follows first order kinetics then the graph of rate of formation (R) of B against time t will be:
(1) 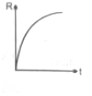 (2)
(2) 
(3)  (4)
(4) 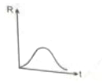
Decomposition of into is first order reaction. Which of the following graph is correct?
(1)  (2)
(2) 
(3)  (4)
(4) 
A gaseous compound A reacts by three independent first order process (as shown in figure) with rate constant for product B, C and D respectively. If initially pure A was taken in a closed container with P=8 atm, then the partial pressure of B (in atm) after 100 sec from starting the experiment,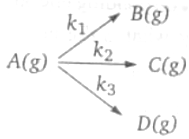
(1) 0.288
(2) 0.577
(3) 1.154
(4) none of these