What is produced when Br2 is added to cis-but-2-ene?
1. Racemic mixture of 2,3-Dibromobutane
2. Meso form of 2,3-Dibromobutane
3. Dextro form of 2,3-Dibromobutane
4. Laevo form of 2,3-Dibromobutane
Major product of the above reaction is:
1.
2.
3.
4.

| 1. |  |
2. |  |
| 3. |  |
4. |  |
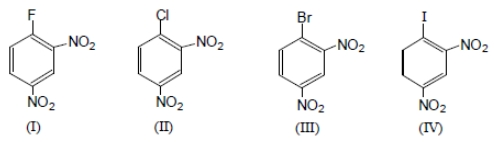
The ease of aromatic nucleophilic substitution among these compounds will be in the order as :
1. IV > I > II >III
2. IV > III > II > I
3. III > II > IV >I
4. I > II > III > IV
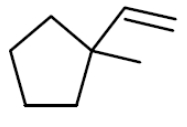
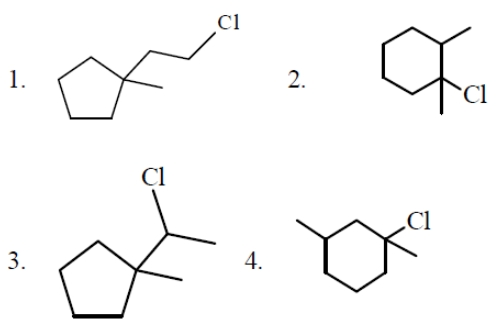
Which haloarenes is most reactive towards electrophilic substitution reaction ?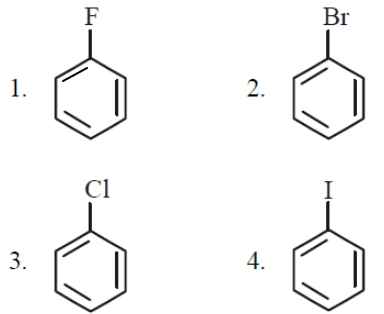
Among the following bromides given below, the order of their reactivity in the SN1 reaction is 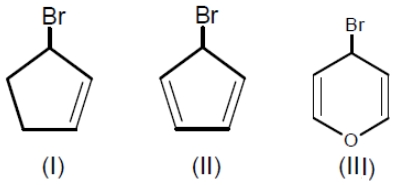
1. III > II > I
2. II > III > I
3. III > I > II
4. II > I > III
On monochlorination of 2-methylbutane, the total number of chiral compounds is
1. 2
2. 4
3. 6
4. 8
Which of the following biphenyls is optically active?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The correct statement about the SN1 reaction is:
| 1. | 100 % racemisation. |
| 2. | Inversion more than retention leads to partial racemisation. |
| 3. | 100 % retention. |
| 4. | 100 % Inversion. |
For the following:
(i) I-
(ii) Cl-
(iii) Br-
The increasing order of nucleophilicity would be:
1. I-<Br-<Cl-
2. Cl-<Br-<I-
3. I- <Cl-<Br-
4. Br-<Cl-<I-
Para-substituted benzyl bromide undergoes SN1 reaction with nucleophiles:
Arrange given four compounds in their decreasing order of reactivity for the above reaction:
1. II > I > III > IV
2. I > IV > III > II
3. I > IV > II > III
4. II > III > IV > I
The major product obtained when is reacted with
Arrange the following in the order of C-Br bond strength in a polar solvent
1. I<II<III<IV
2. III<IV<I<II
3. IV<III<II<I
4. II<I<III<IV
P-chloro toulene is reacted with liquid then the product formed is
1. o-toulidine
2. m-toluidine
3. p-toluidine
4. Both (1) & (3)