
The IUPAC name of the above mentioned compound is -
1. 1,2,3-Tricyanopropane
2. Propane-1,2,3-tricarbonitrile
3. 1,2,3-Cyanopropane
4. Propane Tricarbylamine
A pair that represents chain isomer is:
1. CH3CHCl2 and ClCH2CH2Cl
2. Propyl alcohol and Isopropyl alcohol
3. 2-Methylbutane and Neopentane
4. Diethyl ether and Dipropylether
Number of monochlorinated products (excluding stereo-isomers) obtained from the given reaction are :
| 1. | 4 | 2. | 5 |
| 3. | 6 | 4. | 7 |
The number of possible isomers of the aromatic compound with molecular formula C7H8O are :
1. 3
2. 5
3. 7
4. 9
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Which of the following is the most stable carbocation
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
In the following compounds,
The order of basicity is
1. IV>I>III>II
2. III>I>IV>II
3. II>I>III>IV
4. I>III>II>IV
What is the decreasing order of stability of the carbocation ions?
1. I>II>III
2. II>III>I
3. III>I>II
4. II>I>III
The correct order of basicities of the following compounds is:
(1)
(2)
(3)
(4)
1. 2>1>3>4
2. 1>3>2>4
3. 3>1>2>4
4. 1>2>3>4
In pyrrole,
The electron density is maximum on
1. 2 and 3 2. 3 and 4
3. 2 and 4 4. 2 and 5
In Duma's method of estimation of nitrogen, 0.35 g of an organic compound gave 55 ml of nitrogen collected at 300 K temperature and 715 mm pressure. The percentage composition of nitrogen in the compound would be:
(Aqueous tension at 300 K = 15 mm)
1. 16.45
2. 27.45
3. 44.45
4. 35.45
Total isomers for are-
| 1. | 4 | 2. | 5 |
| 3. | 7 | 4. | 8 |
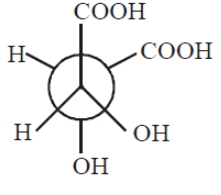 has the configuration
has the configuration
1. R,R
2. S,S
3. R,S
4. May be 1 or 2
Which hydrocarbon is most acidic?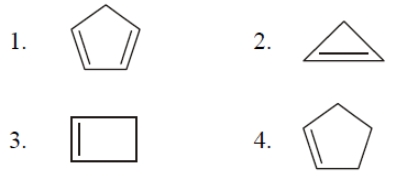
The aromatic compound is 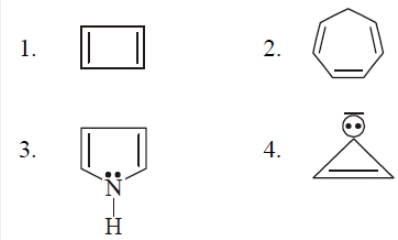
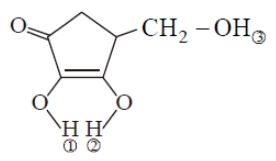
In the above compound, the correct order of hydrogen as is
1. 1>2>3
2. 2>3>1
3. 2>1>3
4. 3>2>1
Consider the following resonating structures of HCOOH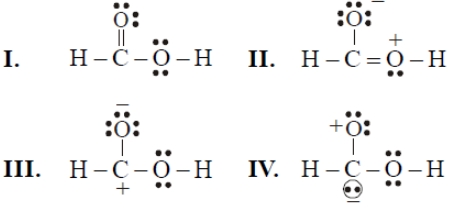
The order of stability is
1. I>II>III>IV
2. IV>I>II>III
3. I>III>II>IV
4. II>I>III>IV
Which compound is most reactive towards electrophilic substitution reaction?
1. Phenol
2. Aniline
3. Methoxy benzene
4. Toulene
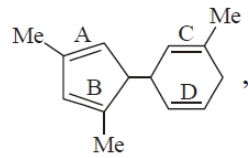
The double bond in the above-mentioned compound that accepts proton fastest is:
| 1. | A | 2. | B |
| 3. | C | 4. | D |
In 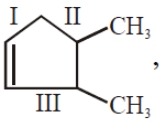 the decreasing order of bond length will be
the decreasing order of bond length will be
1. I>II>III
2. II>III>I
3. III>II>I
4. II>I>III