A hydrocarbon has molecular formula . In this hydrocarbon only one molecule can be added. On ozonolysis it gives symmetrical diketone. The hydrocarbon is
1.
2.
3.
4. All of these
How many halogen derivatives are possible for ?
1. 2
2. 4
3. 6
4. 9
The order of melting point for isomeric pentanes is
1. n-pentane > Isopentane > Neopentane
2. Neopentane > Isopentane > n-pentane
3. Neopentane > n-pentane > Isopentane
4. Isopentane > n-pentane > Neopentane
The compound ‘A’ is
1.
2.
3. Both (1) and (2)
4. Neither (1) nor (2)
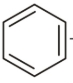 +
+
the compound ‘A’ is
1.
2.
3.
4.
A (Major)
l
Br
the compound ‘A’ is
1.
2.
3.
4. No Reaction
Compound ‘A’ and ‘B’ respectively
1. Both are Cis-butene-2
2. Cis-butene-2, Trans-butene-2
3. Both are Trans-butene-2
4. Trans-butene-2, Cis-butene-2
The absolute configuration of the following compound is
1. 2 S, 3R
2. 2 S, 3 S
3. 2 R, 3S
4. 2 R, 3R
The correct order of solvolysis in the following alkyl halide is
(1)
(2)
(3)
(4)
Bottles containing and lost their original labels. They were labelled A and B for testing. A and B were separtely taken in test tube and boiled with NaOH solution. The end solution in each tube was made acidic with dilute and some solution added. Solution B gave a yellow ppt. which one of the following statements is true for the experiment ?
(1) Addition of was unnecessary
(2) A was
(3) A was
(4) B was
Replacement of ‘Cl’ of chlorobenzene to give phenol requires drastic conditions but chlorine of 2,4-dinitrochloro benzene is readily replaced because
1. makes the ring electrons rich at ortho and para position
2. withdraws electrons from meta position
3. donates electrons at m-position
4. withdraws electrons from ortho and para positions
Among the following bromides given below, the order of their reactivity in the reaction is
1. III > II > I
2. II > III > I
3. III > I > II
4. II > I > III
Which haloarenes is most reactive towards electrophilic substitution reaction ?
(1)
(2)
(3)
(4)
The ease of aromatic nucleophilic substitution among these compounds will be in the order as:
1. IV > I > II > III
2. IV > III > II > I
3. III > II > IV > I
4. I >II > III > IV
Arrange the following in the order of C-Br bond strength in a polar solvent:
| (I) |  |
(II) |  |
| (III) |  |
(IV) |  |
1. I < II < III < IV
2. III < IV < I < II
3. IV < III < II < I
4. II < I < III < IV