The reaction intermediate involved in the addition of \(\mathrm{HBr}\) to propene in the absence of peroxides is:
| 1. | \(H^+\) | 2. | \(Br^-\) |
| 3. | \(H \) | 4. | \(Br\) |
Reductive ozonolysis of the alkene, will give:
1. Only
2. Only
3. Only
4. Mixture of
The main product A and B in the above mentioned reaction are respectively-
1.
2.
3. 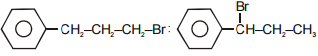
4. 
The structure of intermediate A in the following reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
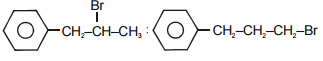
The reaction of C6H5CH =CHCH3 with HBr produces:
| 1. | \(C_{6} H_{5} \underset{Br}{\underset{\left|\right.}{CH}} CH_{2} CH_{3} \) |
| 2. | \(C_{6} H_{5} CH_{2} \underset{Br}{\underset{\left|\right.}{CH}} CH_{3} \) |
| 3. | \(C_{6} H_{5} CH_{2} CH_{2} CH_{2} Br \) |
| 4. |  |
Sabatier Senderen’s reaction is an example of-
1. Non thermic reaction
2. Exothermic reaction
3. Endothermic reaction
4. None of the above
Reaction of alkene and peracid gives oxirane. This reaction is named as-
1. Peroxidation
2. Oxidation
3. epoxydation
4. None
The oxidation of benzene by V2O5 in the presence of air produces:
| 1. | Benzoic anhydride | 2. | Maleic anhydride |
| 3. | Benzoic acid | 4. | Benzaldehyde |
The product B in the above-mentioned reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Cyclopentadiene is much more acidic than cyclopentane, because:
| 1. | Cyclopentadiene has conjugated double bonds. |
| 2. | Cyclopentadiene has both sp2 and sp3 hybridized carbon atoms. |
| 3. | Cyclopentadiene is a strain-free cyclic system. |
| 4. | Cyclopentadienyl anion ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability. |