Which of the following bromides is the major product of the reaction shown below, assuming that there are no carbocation rearrangement?
1.
2.
3.
4.
End product of the reaction is:-
(a)
(c)
Which one of the following compounds gives acetone (CH3)2C=O as one of the products of
1.
2.
3.
4.
What is the major product expected form the following reaction?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Addition of OsO4 to cyclopentene generates:
(1) Achiral product
(2) Racemic product
(3) Meso product
(4) Optically active product
Compound (C) in above sequence of reaction is:
1. 
2.
3.
4.

Product (C) of the above reaction is :
1. 1,3-Hexadiene
2. 1,4-Pentadiene
3. 1,3-Butadiene
4. 1,3-Heptadiene
Which reagents are suitable to accomplish the mentioned conversion?

1. O3/H2O2, HO-/
2. HBr, alc. KOH, O3, LiAlH4, H+/
3. HBr, t-BuOK, O3, KMnO4,
4. HBr, alc. KOH, O3, KMnO4,
H-CC-Ph 
| 1. |  |
2. |  |
| 3. | \(\mathrm{Ph}-\mathrm{C} \equiv \mathrm{C}-\mathrm{I}\) | 4. | \(\mathrm{I}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}\) |
The degree of unsaturation in compound (A) is -
(1) 7
(2) 8
(3) 9
(4) 10
Which of the following compounds is the slowest to react with nitrosonium ion (NO+)?
1.
2.
3.
4.

Product (C) is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

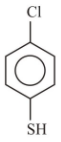
1. 
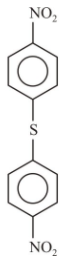
2. 
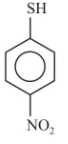
3. 
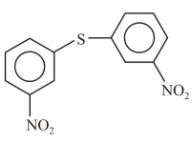
4. 
n-Butylbenzene on oxidation with hot alkaline KMnO4 gives:
| 1. | Benzoic acid | 2. | Butanoic acid |
| 3. | Benzyl alcohol | 4. | Benzaldehyde |
Which of the following compounds undergoes bromination of its aromatic ring (electrophilic aromatic substitution) at the fastest rate?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
An alkene reacts in accordance with Markownikoff's rule to give a product of 1-chloro-l-methylcyclohexane with HCl. The possible alkene is:
| 1. |  |
| 2. |  |
| 3. | Both 1 and 2 |
| 4. |  |
The products that formed when the following compound is treated with Br2 in the presence of FeBr3 are:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
In a reaction of aniline a coloured product C was obtained.
The structure of C would be
(a)
(b)
(c)
(d)
Reaction of alkene and peracid gives oxirane. This reaction is named as-
1. Peroxidation
2. Oxidation
3. epoxydation
4. None
The most probable mechanism for the above reaction is-
1. E1
2. E2
3.
4. elimination